CAR-T Therapeutic Direction of Lung Cancer Therapy
페이지 정보
Author HK HIS Date19-09-16 18:19 View269 Comment0관련링크
본문
The CRI Cancer Research Institute has published a report on the development of global tumor immunotherapy trends in Nature Reviews Drug Discovery. In a one-year study, the number of global programs for tumor immunity therapy increased by 67%, target research by 50%, and for companies and government agencies participating in clinical development programs, increased by 42% It turns out that much research has been concentrated on treatment.
In 2011, approval of ipilimumab as a treatment for melanoma began with a revolutionary event in tumor immunotherapy, changing the paradigm of cancer treatment. Up to now, eleven new tumor immunotherapies have been approved and become the treatment standard for various cancers.
The worldwide Oncology Immunization Program is growing at a rapid rate of 67%, and it is possible to divide the immunotherapy drugs into 6 types depending on the mechanism of action. Targeted T cell immunomodulator(E. G., A monoclonal antibody to PD1 or CTLA4), other immunomodulators (e. G., A stimulant of TLR or interferon alpha-beta receptor 1 (IFNAR1)), a tumor vaccine (E. G., BCG) vaccines), cell therapy (e. G., Impedance antigen receptor (CRA) or T cell receptor (TCR T cell therapy), polyp virus(T-Vec). Among the six types of immunotherapy, cell therapy was the fastest rate of 113%, while the polyp virus study was found to be slow at 16%. In addition, it is the most studied technique, and the number of ongoing programs is 864, accounting for 25% of total tumor immunity programs.
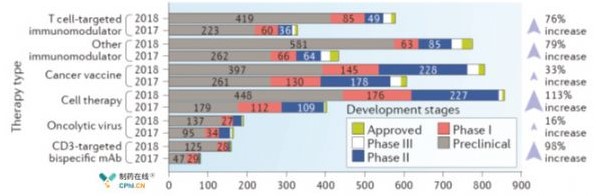
Fig. 1 Trends in the Immunotherapy Program until September 2018
Currently, 417 targets are under study in the global tumor immunotherapy program. Over the past year, research on immunotherapy targets has increased by 50%. Interestingly, half of the research and development (R & D) in 2017 focused on the top 23 targets, but in 2018 we focused on the top 48 targets. Drugs are usually approved for one target, reducing the number of R & D projects involved. For example, despite the 113% increase in cell therapy in 2018, CD19-targeted anti-cell therapy increased only 37%. On the other hand, the study of drugs (targets identified by bioinformatics analysis of individual patient tumors) whose targets are added to new antigens has increased by 133% over a year. Tumor Immune Therapy An increase in the number of target tumors will result in more immunotherapy in the future It will be the basis for approval. Notably, the actual number of nonspecific tumor-associated antigen (TAA) drugs is decreasing, indicating that the area is developing in a more precise direction.
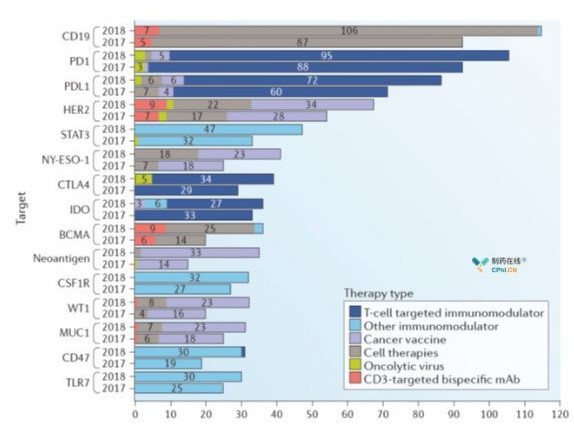
Fig. 2 Immunotherapy TOP15 Target
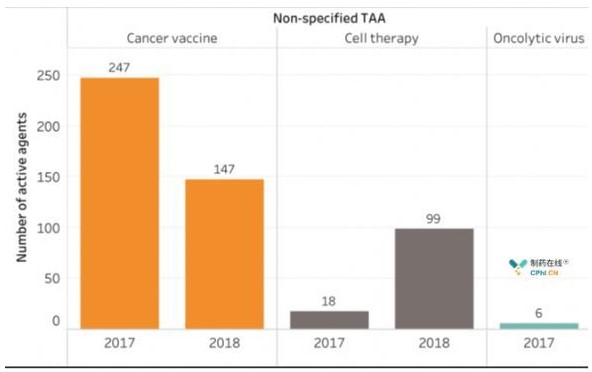
Fig. 3 Nonspecific Tumor Associated Antigen Item Trends
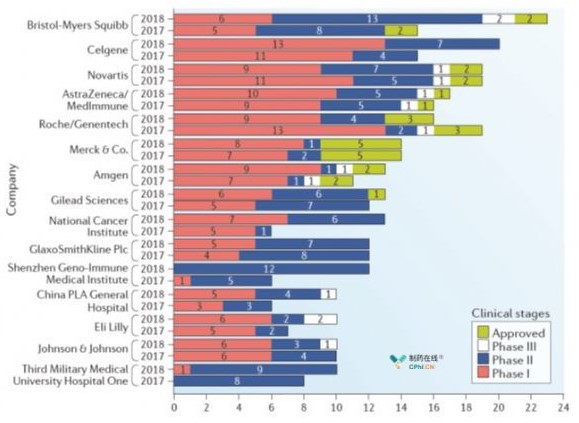
Fig. 4 TOP15 Immunotherapy Companies and Institutions





