Immunotherapy, which has a 89% response rate to cancer cells, sets the FDA's new standard. In recent years, new technologies for tumor therapy have grown at a very rapid pace with various drugs and technologies. It is news about TIL treatment which is new immunotherapy that we introduced recently. Recently, the FDA has been approved for LN-145, a treatment for tumor invasive lymphocyte (TIL) cells. This is a hopeful news for patients with immunotherapy for tumor cells. FDA approved LN-145 cell therapy. The FDA's approval is based on an ongoing trial of innovaTIL-04 (C-145-04), which resulted in an overall response rate (ORR) of 44% for TIL treatment in end-stage cervical cancer patients. On February 4, 2019, the last patient to evaluate the data is 27 patients.
0 44% (12 patients) Patient effective outcome 1 response partial response 9 patients unreacted 2 patients were effective.
0 The disease control rate was 89%.
0 The median follow-up period was 3.5 months and there was a response rate of 11 out of 12 patients.
0 There were no serious side effects.
The results of this study were presented at the ASCO Congress in June 2019.
The FDA has approved the use of LN-145 treatment based on results that are effective in treating end-stage cervical cancer patients. This means that cervical cancer patients treated during or after conventional chemotherapy have a response rate of 4% to 14%. The newly developed LN-145 therapy is a very stable cell therapy with a response rate of 44% for terminal cervical cancer patients. This new cell-based immunotherapy has enabled long-term disease control, the researchers say. I hope that more patients will be applied to the treatment as a complement to this treatment.
What treatment is LN-145?
The treatment called LN-145 is very new. However, LN-145 is based on TIL therapy, which is one of the immunotherapies. LN-145 is a therapy developed by the lovance company. It modifies the T cell receptor (TCR) The cells are injected back into the patient to induce autoimmune action.
Immune cells with the strongest killability
TIL (Tumorinfiltrating Lymphocytes) refers to lymphocytes infiltrating the tumor. Most of the surgically resected tumor tissues are tumor cells and the lymphocyte fraction is small. Some of these lymphocytes are T-cells for tumor-specific mutations in the tumor, but the function of the tumor is inhibited by some causes (for example, the production environment of the tumor, PD-1) The tissue will be produced. However, the researchers investigated whether some of these tumor tissues could be more effective when stimulated with certain types of lymphocytes, activated and reintroduced into patients through some in vitro culture methods.
How does TIL treatment differ from other immunotherapy methods?
TIL and first-generation immunotherapy, eg CIK, have different points.
1. Immune cell principle is different. TIL immune cell therapy comes from cancer tissues and CIK is found in blood.
2. The direction of the next-generation TIL therapy is to isolate immune cells that are targeted only to tumor cells during screening.
TIL Treatment Course
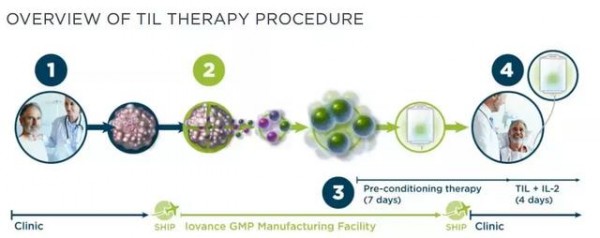
1. First, the tumor is removed from the patient's body through surgery or surgery.
2. The tumor sample is sent to the GMP facility where it separates the TIL and cultivates billions of TILs for three weeks.
3. The patient begins a week of preliminary treatment and receives primary TIL cultured cells.
4. Patients TIL infusion + IL-2 is activated and activated by TIL in the patient's body.
The researchers first identify specific mutations in the patient's case, then use mutation information to find T cells that can most effectively target these mutations, and then extract and mutate cell-mutant T cells from the patient's tumor. These immune cells are cultured in vitro and injected into the patient again. At the same time, it injects the immune enhancer, white cell spot 2 (IL-2), or the recently developed PD-1 inhibitor Keytruda, in order to maximize the effect of both immunotherapy and tumor suppression.
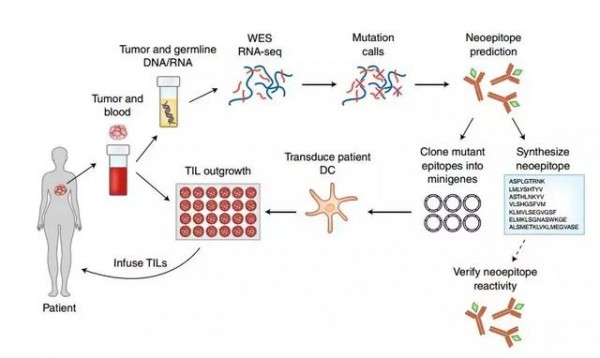
Research procedure (Source: Nature Medicine)
Based on the procedure described above, this new treatment has been developed to be "customized" for the patient.
Cervical cancer
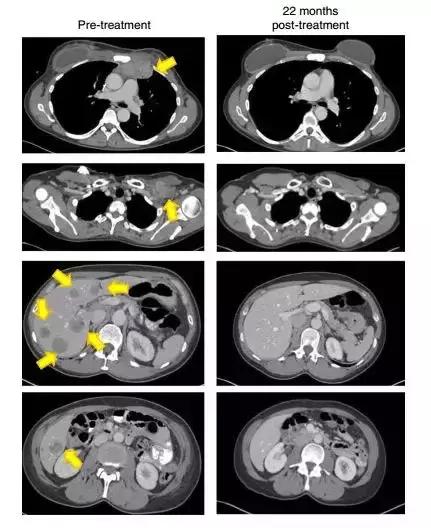
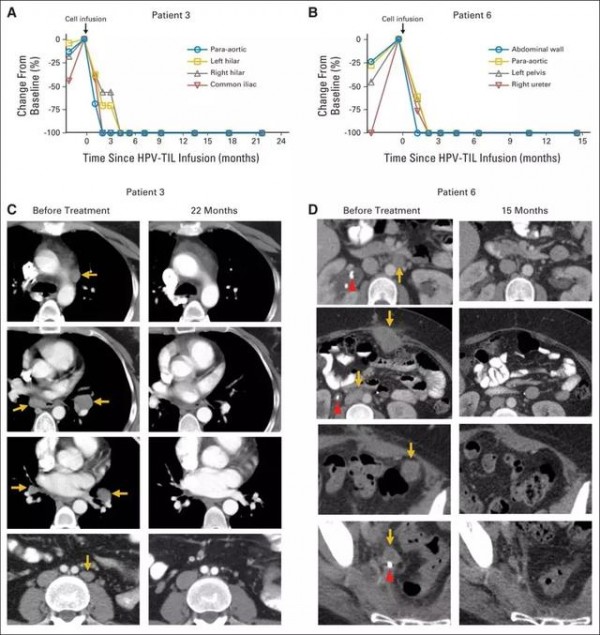
A study by the National Institutes of Health (NIH) states that TIL therapy has a sustained response, including complete recall of metastatic cervical cancer. Patients with metastatic squamous cell carcinoma underwent various treatments, including platinum-based therapy and radiotherapy, but both lungs at the sides of the aorta showed metastasis of the lower part of the skull and metastasis to the iliac area (Figs. 1A and 1C). (Fig. 1A and 1C) Another metastatic cancer patient has developed into a metastatic metastasis of peritoneal metastases after primary chemotherapy, where the treatment was ineffective. After TIL treatment (Figures 1B and 1D), the tumor was reduced in size at the tumor site and clinical relief was noted.
Breast cancer
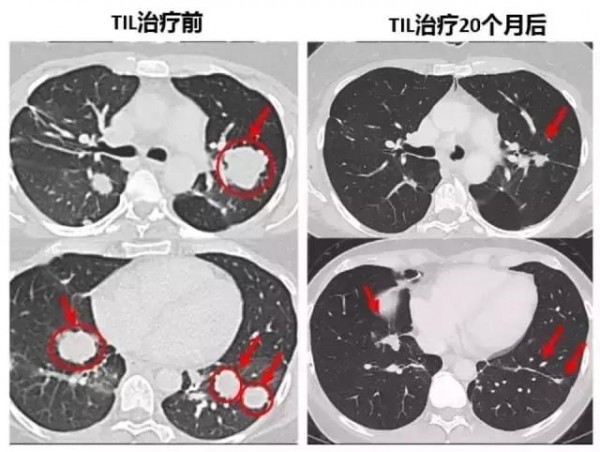
In Nature Medicine's study announcement, we recently reported that patients who were unresponsive to other therapies were miraculously cured by TIL immunotherapy. This is the first time T cell immunotherapy has been applied to end stage breast cancer patients.
After 22 months, the patient's tumor (yellow arrow) disappeared without trace (Source: Nature Medicine)
Rectal cancer
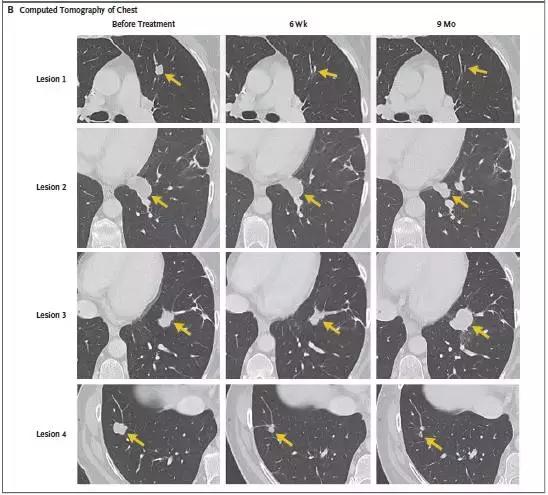
After metastatic colorectal cancer patients underwent TIL cell therapy, 7 lung metastases were either smaller or disappeared.
Bile duct cancer
Patients with end stage cholangiocarcinoma metastasized after multifocal multiple metastasis and were clinically cured after treatment with TIL cells.
Immuno-oncology has just begun to be realized. The method we have found is very limited in part, and more research will reveal more in detail how to control the immune response. These treatments will be solidified through numerous clinical data, and you can look forward to everyday life with advanced treatment techniques.
HK HIS is committed to providing you with the most advanced and up-to-date treatment options.